Case Study
Platinum Crucibles
Because of high melting temperature, oxidation and corrosion resistance, platinum crucibles are key tools for wide range of analytical procedures like dissolving in hot hydrofluoric acid, loss on ignition, ash testing and fusion of slag, mineral, cement and glass samples.
Pure platinum is the basic material choice, offering relatively low price, on the other hand is quite soft and vulnerable to grain coarsening after prolonged high temperature exposure. Alloying with rhodium increase the material hardness and slows down grain growth, but can be significantly more expensive.
Grain Stabilised Platinum
Grain Stabilised Platinum (GSP) is a powder-metallurgy material alternative with introduced dispersed zirconia oxide nanoparticles in the platinum matrix. This yields superior creep resistance and long grain-boundary path for potential “platinum-poison” contaminant, without costly alloying elements. This article aims to compare the above-mentioned materials with the respect to handling experience and structural changes.
Experiment
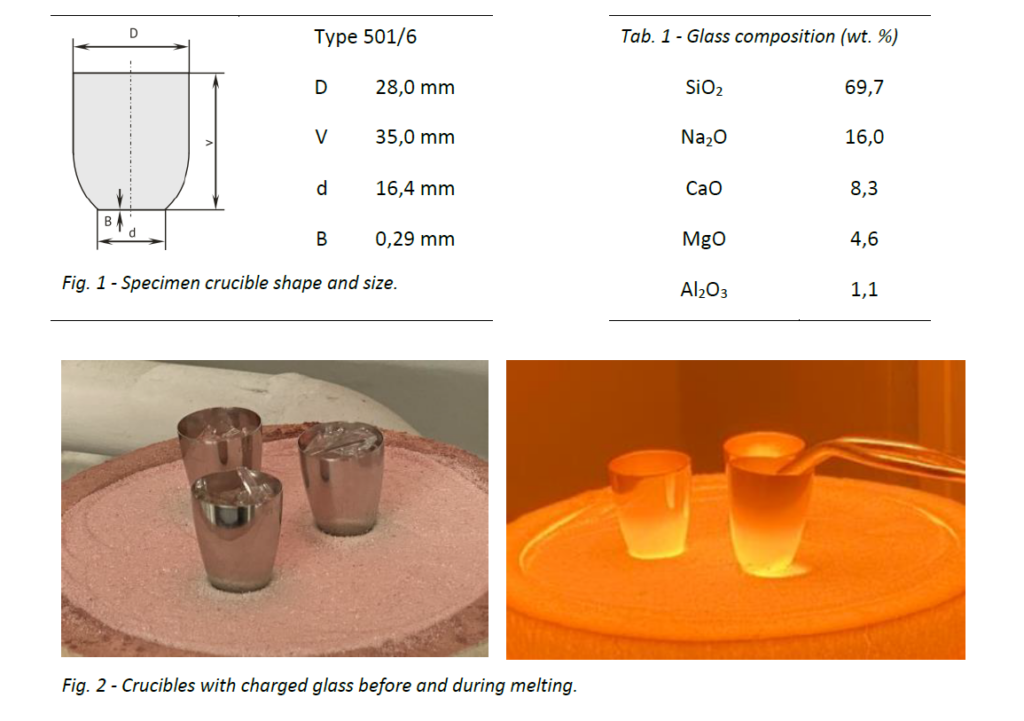
Two sets of 20 ml crucibles (Fig. 1) were manufactured by metal-spinning and polishing from Pt, PtRh10 and GSP metal sheets.
Set A was annealed in air at 1500 °C (ramp 10 K/min, 1.5 h soak, quenched), in order to evaluate the softening (Vickers Hardness 1 kgf) and microstructure changes (optical and laser confocal microscopy).
Set B was used to melt “float glass” samples (Tab. 1), to evaluate chemical interactions and resistance to deformation inflicted by handling.
- Crucibles with glass shards (20 g) were inserted in 800 °C furnace and heated to 1500 °C in air (10 K/min). Another 10 g of material were added to each crucible and left at 1500 °C to soak for another 1 h.
- Molten glass was poured in steel moulds to form blocs (approx. 20 g) for XRF analysis and tempered at 560 °C for 1 hour.
- Crucible walls were quenched in water. After cooling to room temperature, solid glass was mechanically extracted by manual bending the crucible walls. This was easily carried out only with GSP crucible, with the other crucibles the process of heating, pouring, quenching and mechanical extraction had to be repeated.
- In order to clean the residual glass, crucibles were filled with NaOH grains and heated in muffle furnace at 400 °C for 90 mins, which was dissolved in water after cooling. Inner surface of crucibles was then examined by SEM-BSE. Note: This step is usually carried out with hydrofluoric acid which is not corrosive to Pt, compared to sodium hydroxide. However, this procedure was chosen as a less toxic alternative with more corrosive potential in order to compare material resistance.
Results
Set A – Annealed
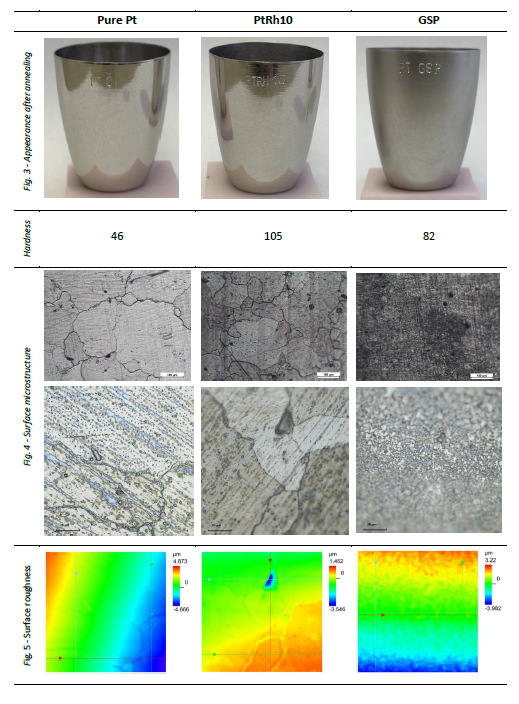
Although annealing did not affect crucible shape, there were apparent changes in surface microstructure. Grains of pure platinum coarsened significantly, in some cases exceeding 500 μm in diameter. Structure of PtRh10 was finer, with grain size about 100 μm (Fig. 4). Surface of both materials was bright and grains boundaries were clearly visible. GSP surface was matte due to presence of thin oxide layer (from residual Zr present in the material) covering grain boundaries – however fine oriented grain texture (not recrystalized) was still apparent (Fig. 3).
Surface roughness (Fig. 5) of Pt and PtRh10 due to grain boundaries is approx. 1 μm, occasionally reaching deeper (observed 3 μm pores). With the absence of grain boundaries, GSP surface roughness is much more homogenous, not exceeding 1 μm.
Regarding mechanical properties, pure Pt got extremely soft and could be easily deformed. PtRh10 was significantly harder, but still could be plastically deformed. GSP crucible on the other hand – even though the hardness was somewhere between other materials – was much stiffer and spring back to original shape after pressing in hand.
Set B – Glass Sample Melting
There was no noticeable contamination (9 ppm ZrO2 difference, standard error 60 ppm) or discoloration of glass sample melted in GSP crucible compared to Pt crucible.
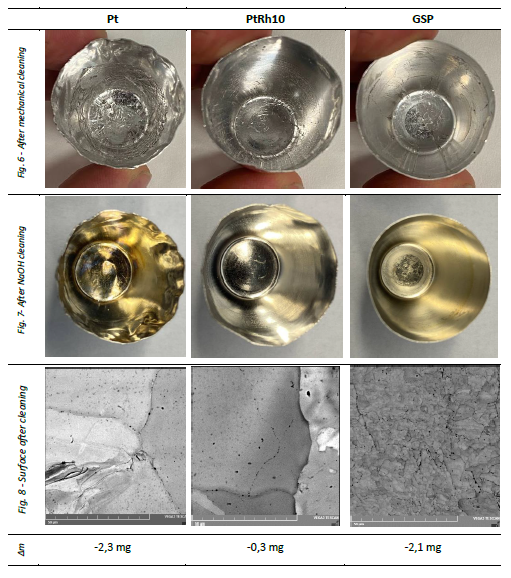
The main difference between materials was the easiness of mechanical cleaning (separation) of remaining glass from the crucible. With the GSP crucible, glass easily fall off after bending the walls and their elastic spring-back to original shape. Both pure Pt and PtRh10 deformed together with broken glass, holding it together and therefore the separation took significantly more effort.
Removal of the residual glass with molten NaOH was successful at all crucibles. The Pt surface was slightly oxidized (yellow discoloration), while PtRh10 took the least damage (Fig. 7) – this corresponds to the lowest observed weight loss after the melting and cleaning procedure, yet in all cases minimal.
Conclusion
- Compared to standard materials for laboratory crucibles (Pt and PtRh10), grain stabilized platinum (GSP) offers improved stiffness and resulting shape retention and easier mechanical cleaning.
- If molten NaOH is used for cleaning, PtRh10 offers best corrosion resistance.
- There is no observable contamination of glass sample by ZrO2 contained in GSP after standard melting procedure.
Acknowledgement
Glass melting procedure, laser confocal microscopy (roughness measurement) and glass XRF analysis was carried out by University of Chemistry and Technology Prague (VŠCHT Praha), dept. of Glass and Ceramics.

